
It is often called LLPS (liquid-liquid phase separation). It can be helpful if you are performing a liquid-liquid extraction and are fearful of emulsions. When you are trying to perform a crystallization or recrystallization LLPS is bad news because it is what we practitioners call 'oiling out'.
As KiloMentor has often repeated, when devising a process, chemists are really guessing when they try to assess how well and how easily they will be able to purify the solid intermediates they need to recover/purify by crystallization. One of the mantras of the KiloMentor blog is: Choose process schemes that incorporate rugged scalable phase switches that either improve purity before a final crystallization or enable process steps to be telescoped to avoid entirely some of these crystallizations.
Having the substance you are trying to crystallize oil out is high on the list of those things you don’t want to happen. This is particularly true on scale because then you are working in a vessel with a stirrer that does not scrape the walls and in which you can’t easily observe what is happening. Because oiling out at scale occurs down inside a poorly illuminated reactor, in situations when that oil eventually solidifies you may never learn what happened. All that may be evident is that the purification failed and the impurities are not uniformly distributed in the product.
Even in the most rugged reaction sequences, successful crystallization of solid intermediates may be required, and reducing the likelihood of oiling out of low-melting solids will be needed to avoid a major dislocation.
Only one article ever accepted by the Journal of Organic Process Research & Development contained ‘oiling out’ in its title [ Jie Lu et al., Org. Process Res. & Dev. 2012, 16, 442-446].
Only three pages in Niel Anderson’s, Practical Process Research & Development, First Edition pertain to oiling out problems in crystallization (Sorry – I can’t afford to pay for both First and Second Editions to check for updates). In the one example on pg. 280 of the first edition, Anderson cites the case of a pharmaceutical product isolation where oiling out is avoided by adjusting processing to make sure that plenty of seeds are available. In this example, the drug captopril was crystallized by first forming a thick seeding suspension comprising some previously isolated captopril solid, acetic acid, and sodium chloride all together in water, and then followed by adding slowly and simultaneously (i) the strongly basic hydrolyzate obtained by first treating S-acetyl captopril methyl ester with 3.3 equivalents of sodium hydroxide and (ii) aqueous HCl; the latter, in such amounts that the crystallizer contents always remained acidic. By forming the captopril in situ in the presence, throughout the entire nucleation, of many preformed captopril crystallites, oil was not formed even though there was a high concentration of sodium chloride in the water.
The oiling-out phenomena can be categorized by two parameters. The first is temperature. Oiling out near or above the solute’s melting point should not be surprising at all. Separation of solid should not be expected if the solution saturation is exceeded at a temperature where that substrate is expected to be a liquid. In this case, the solution is too concentrated for work at that temperature. More serious is the oiling out that occurs near the melting point of the main solute and most worrisome is the oiling out that occurs below that melting point.
The second parameter pertains to solvents. There is oiling out from a single solvent or from a solvent combination. It seems to me that oiling out from a single solvent below the anticipated melting point of the substrate most often arises simply because the rate of phase separation is faster than the rate of nucleation. The antidotes should be one or both slower cooling and seeding.
Oiling out from a solvent mixture appears more frequently and has a more obvious explanation. The separating solute can cause the solvent combination to demix and separate. This situation will be most common when the solvent mixture is composed of solvents of quite different polarities; for example ethanol-hexane.
Another scenario can play out when the main impurities begin to separate in preference to the desired product and they contaminate the emerging product enough to reduce its melting point below the solution temperature. This is likely to arise when trying to purify a main substance with more polar impurities by crystallizing from a strongly apolar solvent or purifying a main substance with predominantly less polar impurities from a strongly polar solvent.
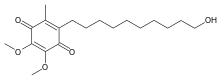 |
| Idebenone |
It would seem to me that this is the situation in the Jie Lu et al. example cited earlier. Idebenone comprises a dialkyl-dimethoxyl-p-quinone with a primary hydroxyl in the side chain. Idebenone is the subject of the Liu paper. The two impurities of concern in it each have one or the other of the two methoxyls demethylated to a phenolic hydroxyl. Thus these impurities are distinctly more polar than idebenone itself, yet this idebenone is being recrystallized from methylene chloride-hexane. I, myself, have extensive experience with idebenone processing. From this experience, I know that it can be recrystallized in high yield from ethanol-water and this would most likely be a preferred method for getting rid of these phenolic impurities without any risk of oiling out.
No comments:
Post a Comment