 |
| A Cryptophenol |
One of the goals of the Kilomentor blog is to reinforce awareness of some of the simple, but robust, methods of isolation which have been used more frequently in the past but that can easily be scaled up.
Claisen’s alkali
is made by dissolving 35 g of potassium hydroxide pellets (itself 85% potassium
hydroxide and 15% water) in 25 ml of water with vigorous stirring and
simultaneous external ice-cooling, followed by dilution with 100 ml of methanol
and repeated cooling. It is necessary to
be thorough with the cooling because otherwise the base will probably react
with the carbon dioxide in the air and weaken itself.
Claisen’s alkali
is a powerful base solution that dissolves organic substrates sufficiently well
so that the rate of deprotonation is rapid. At the same time, the liquid is
immiscible and unreactive with saturated hydrocarbon solvents. Weak hydrophobic
phenols and enols can be extracted out of hydrocarbon solution by contacting
them with Claisen’s alkali. Thus, for example, 2,4,6-triallylphenol, which is
insoluble in aqueous alkali can be extracted from petroleum ether with Claisen’s
alkali. As another example, Vitamin K1 is easily isolated from 3-5% alfalfa concentrate
by shaking an alcoholic suspension of the oil with aqueous sodium hydrosulfite
to reduce all quinones to the hydroquinone state, then extracting with
petroleum ether to take up all the hydrophobic solutes and then extracting this
with Claisen’s alkali. The extract is
yellow from the formation of potassium anions and dianions. By dilution of the
alcoholic solution with water even without acidification the K1 hydroquinone
can be extracted back into ether and isolated from there. The back extraction into ether works even
without acidification because the hydrophobic hydroquinone in aqueous solution
hydrolyzes enough to be taken up into ether and this drives the hydrolysis
according to Le Chatelier’s Principle.
Lipophilic primary
and secondary sulfonamides can also be separated from other substances that lack weakly acid hydrogens using Claisen’s alkali extraction of a pet. ether
solution. The key is that the
sulfonamides must be sufficiently large and hydrophobic to dissolve in the pet.
ether.
There is no study
that I know that teaches what compounds can be taken up in Claisen’s alkali. At
the very least it seems that a hydrogen on carbon needs to be activated by at
least one carbonyl and one double bond.
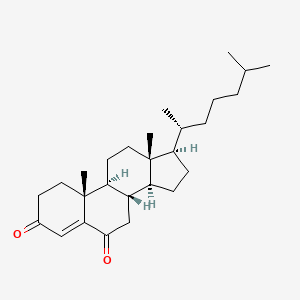
In another well-known example exhaustive dichromate oxidation of
cholesterol and removal of extensive acidic fractions by a simple aqueous base
extraction leaves a mixture of delta 4 cholestene-3,6-dione and several
monoketones and other neutral products (Org. Syn. Coll. Vol. 4, 189 (1963)).
Repeated extraction of a solution of this mixture in pet. ether with Claisen’s
alkali as long as the extracts are colored yellow (note the simple visual test
of effectiveness) affords a simple means of isolating the total enedione
present, which in this case is 40%.
Compounds which
are so weakly acidic that they cannot be extracted by basic aqueous solution
but which can be captured into Claisen’s alkali are termed cryptophenols, a
descriptive term that is not however widely known or used. Molecules in which a
methylene is substituted by two moderately electron-withdrawing groups would be
candidates for Claisen’s alkali extraction.
No comments:
Post a Comment