A major element of Kilomentor’s philosophy of
organic synthesis and chemical process development is to recommend developing
processes that have as preferred process intermediates compounds that are
sufficiently acidic or basic that they can be separated and purified by
acid-base extraction or which can be readily crystallized in salt form.
In further examining the latter aspect, an important
question is what salt of amines, in general, is most likely to be insoluble in
water. Presented with a previously
unknown amine-free base and asked to prepare a solid salt that can be readily
purified, is there any salt that is more likely to be made successfully on the
first attempt? Although modern chemists
realize that any relationship between molecular structure and physical
properties is tenuous, Kilomentor is convinced that the pamoate is that salt.
The acid, alternately called pamoic acid, embonic
acid or methylene-bis-1-(2-hydroxy-3-naphthoic acid was first described by
Hosaeus in 1892! In 1929, I.G.
Farbenindustrie A.G. patented a method for manufacturing sparingly soluble, tasteless salts of nitrogenous basic compounds, in particular salts of
alkaloids such as quinine and strychnine, and of bases of the ‘plasmochin’ type, using embonic acid. In 1946, a US patent US 2,397,903 was issued, claiming a
methylene-bis-2-hydroxy-3-naphthoic acid salt of a compound of the class
consisting of thiamine and pyridoxine.
From the very beginning of its application in
chemistry the substance called embonic or pamoic acid was used to prepare water
insoluble salts of amines, particularly amines that have two basic functional
groups.
Pamoate is a pharmaceutically acceptable
salt. Thus, trace residues are not
problematic in drug synthesis. Pamoates have been used frequently to make drug
products. It is almost
exclusively used to make modified release formulations that draw out the active
substance’s delivery into the bloodstream of a patient and so provide a long-lasting medical effect. This extended release relies on the poor relative solubility of pamoate salts in digestive fluids.
Pamoic acid is commercially available but its
synthesis is also simple and inexpensive. The procedure were provided by Barbier
and Gaimster in J. Appl. Chem., 2, October 1952. Since this old reference is probably stored
in an off-site warehouse even in one of the best libraries, Kilomentor is
reproducing the synthesis procedures below:
“Method I.
2-hydroxy-3-naphthoic acid (750 g.) was
suspended in glacial acetic acid 97.5 l.) and stirred at 95-100 C until
dissolved. A mixture of glacial acetic acid (750 g.), 40% formaldehyde solution
(450 g.) and concentrated sulfuric acid (71 g.) was added over 20 minutes, the
reaction being sufficiently exothermic to maintain the temperature between 95
and 100 C. The suspension of embonic
acid was stirred at 95-100 C for 30 minutes, allowed to cool to 70 C, filtered
and washed first with hot glacial acetic acid 94.5 l. and then distilled water
until the washings were no longer acidic to Congo red. The material was dried
at 100 C to give embonic acid (700 g.).
Method II.
2-hydroxy-3-naphthoic acid (500 g.) and
10% sodium hydroxide (1500 ml.) was heated to 90 C with stirring; about two
thirds of the solid dissolved. 40% formaldehyde solution (63 g.) was added, the
temperature rising to 92 C, then a further 83 g. of 40% formaldehyde solution,
which caused a further rise to 95 C. No solid remained at this stage. After
heating at 95 C for a further 5 minutes the solution crystallized
spontaneously. The mixture was maintained at 95 C for one hour, cooled to 20 C and the sodium embonate filtered and
washed with saturated brine (125 ml.).
the damp sodium embonate (about 1.2 kg.) could be used as such or
converted to the acid by dissolving in a mixture of water (3 l.) and acetone
(700 ml.), by heating to 50 C and adding glacial acetic acid (225 ml.) and then
concentrated hydrochloric acid (about 200 ml.) until the mixture was acid to
Congo red.
The precipitated embonic acid (480 g.) was
filtered, washed with hot water until free of chloride, and dried at 100 C.”
The above-noted paper by Barbier and Gaimster
provides other useful information. It teaches that a solution of sodium
embonate can readily be prepared by dissolving embonic acid in an aqueous
solution of sodium hydroxide and although the equilibrium solubility of sodium
embonate in water at 20 C is less than 10%, the solution readily supersaturates
and stronger solutions can easily be prepared.
The salts with thiamine and pyridoxine are formed as follows.
US2397903
Thiamine
Example I.- To a solution of 17 parts of thiamine
hydrochloride in 200 parts of water was added, with agitation and at room
temperature, a solution of 25 parts of the di-sodium salt of
methylene-bis-2-hydroxy-3-naphthoic acid (?% pure). A cream-colored precipitate, which on
standing partly crystallized, formed.
After recrystallization from 70% ethanol, a product, which decomposed at
180-185 C, was obtained. The yield was almost theoretical. The product had a
biological potency equivalent to the thiamine and may be termed
thiamine-methylene-bis-2-hydroxy-3-naphthoate.
Pyridoxine
Example II.
To a solution of 41.1 parts of
pyridoxine hydrochloride in 500 parts of water was added with agitation and at
room temperature, 43.2 parts of the disodium salt of methylene-bis-2-hydroxy-3-naphthoic
acid. An amorphous precipitate, which on standing partly crystallized, formed.
It was recrystallized from acetone. On heating, it decomposed. It was a little
more soluble in water than the product of Example I. It was soluble in aqueous
ethanol and acetone. It had a biological potency equivalent to the pyridoxine
hydrochloride.
There are other examples from other patents.
WO9425460A1
Risperidone
Example I
A solution of 3- [2- [4-(6-fluoro-
1,2-benzisoxazol-3-yl)- I-piperidinyl) ethyl]
-6,7,8,9-tetrahydro-2-methyl-4H-Pyrido[1,2-ajpyrimidin-4-one,19.70 g (0.
048mol) in ethanol (600ml) was added to a solution 18.64 g of pamoic acid (0.
048mol) in N,N-dimethylformamide (400ml). (1g/22 ml )
The mixture was stirred for 3 hours. The resulting precipitate was filtered off
by suction, washed with ethanol and dried, yielding 3 1 g (8.1 %) of
3-[2-[4-(6-fluoro- 1,2benzisoxazol-3-yl)- I -piperidinyl)ethyl)
-6,7,8,9-tetrahydro-2-methyl-4H-pyfido[ 1, 2ajpyrimidin-4-one
4,4'-methylenebis[3-hydroxy-2-naphthalenecarboxylate) (1: 1); mp. 269.2'C.
This
is a very poor yield of salt; just 8.1%.
Pamoic acid apparently is soluble in dimethylformamide. This is useful
information. The risperidone was dissolved in the usual ethanol. Perhaps the experimentalist did not wait long
enough for the solid to all precipitate. They filtered after 3 hours.
WO05016261A2
Haloperidol
and Aripiprazole
Example
1:
The pamoate salt of haloperidol can be prepared by treatment of haloperidol
with pamoic acid or pamoate salt in solvent. Haloperidol pamoate can be
prepared by adding a solution of haloperidol in an appropriate solvent, ea.
ethanol with acetic acid, to a solution of disodium pamoate, pamoic acid or
other pamoate salt and leaving undisturbed for 1-3 or more days until
precipitation. Alternatively, other methods such as evaporation, slow or fast
cooling or stirring solutions can also be used to precipitate salt.
Specifically, 2.5 ml of a 0.1M solution of haloperidol in an acidified ethanol
(5% acetic acid) was added to 2.5 ml of a 0.1M solution of disodium pamoate
(2.5ml) in ethanol/water (50/50 v/v). The mixture was allowed to sit at room
temperature for 1-3 days. The resulting precipitate was filtered off by
suction, washed with ethanol and dried in a vacuum oven at 60°C, yielding 240 mg
of 1:1 haloperidol pamoate salt.
Example 2:
2.5 ml of a 0.25M solution of haloperidol in an acidified ethanol (5% acetic acid) was added to 12.5 ml of a 0.05M solution of disodium pamoate in ethanol/water (75/25). The mixture was allowed to sit at room temperature for 1-3 days. The resulting precipitate was filtered off by suction, washed with ethanol and dried in a vacuum oven at 60°C, yielding 206mg of 2:1 haloperidol pamoate salt
Example 3:
2.5 ml of a 0.25M solution of haloperidol in
an acidified ethanol (5% acetic acid) was added to 6.25 ml of a O.1M solution
of disodium pamoate in ethanol/water (50/50). The mixture was allowed to sit at
room temperature for 1-3 days. The resulting precipitate was filtered off by
suction, washed with ethanol and dried in a vacuum oven at 60°C, yielding 264mg
of 2:1 haloperidol pamoate salt. - 1 1
Example 4:
ml of a 0.05M solution of haloperidol in
an acidified ethanol (5% acetic acid) was added to 1 ml of a 0.25M solution of
disodium pamoate in ethanol/water (50/50). The mixture was allowed to sit at
room temperature for 1-3 days. The resulting precipitate was filtered off by
suction, washed with ethanol and dried in a vacuum oven at 60°C, yielding 107
mg of 1:1 haloperidol pamoate salt.
Example 5:
5.ml of a 0.05M solution
of haloperidol in an acidified ethanol (5% acetic acid) was added to 2.5 ml of
a O.1M solution of disodium pamoate in ethanol/water (50/50). The mixture was
allowed to sit at room temperature for 1-3 days. The resulting precipitate was
filtered off by suction, washed with ethanol and dried in a vacuum oven at
60°C, yielding 119 mg of 1:1 haloperidol pamoate salt.
Example 6:
A (0.05 - 0.5M) solution of aripiprazole
in an acidified ethanol is added to a (0.05 - 0.5M) disodium pamoate solution
in a mixture of water/ethanol (100/0 0/100). The mixture is allowed to sit at
room temperature for 1-3 days. The resulting precipitate is filtered off by
suction, washed with solvent and dried in a vacuum oven at 60°C.
These
methods teach the method of adding the base acidified with 5% acetic acid in
ethanol to the disodium pamoate in ethanol/water. The disodium salt is more soluble and so this
method depends upon the acidification of sodium pamoate with acetic acid to
create the pamoic acid in situ where it can interact with the amine in the
presence of acetic acid. The more
insoluble amine pamoate crystallizes.
These examples illustrate the fact that pamoates often must be allowed
to change form from a gel like form to crystalline over some time. Heating sometimes accelerates this change.
WO04017970A1
AGN-2979
(C)
Preparation of 3-(3-methoxyphenyl)-3-(3-
dimethylaminopropyl]-4,4-dimethyl-piperidine-2,6-dione pamoate salt (anhydrous)
A solution of AGN-2979 bisulphate salt
obtained in Step B (1 mmole, 430 mg) in 10 ml of water was mixed with methylene
chloride (20 ml) and basified with aqueous ammonium hydroxide (29% w/w). After
separation of the layers, the aqueous phase was extracted twice with methylene
chloride. The combined organic phases were dried over anhydrous magnesium
sulphate and the solvent was evaporated under reduced pressure. The residue was
dissolved in ethanol (10 ml) and mixed with a hot solution of pamoic acid
(embonic acid, 390 mg,1 mmole) in hot ethanol (30 ml) and the mixture was
heated to reflux. After cooling, the pamoate salt crystallised and the salt was
recrystallised in hot ethanol to give a pale yellow powder (melting point =
146°-150°C.
The
procedure separates free base, evaporates to an oil and dissolves it in
ethanol. It is mixed with a hot solution of pamoic acid dissolved in hot
ethanol. The embonate came out in
crystalline form on cooling. This could be useful to effect isolation of a base that should be solid but refuses to solidify for crystallization. It can be first converted to a solid embonate and then back to a purer free base.
WO05075454A2
FORMS
OF
4-(4-METHYLPIPERAZIN-1-YLMETHYL)-n-[4-METHYL-3-(4-PYRIDIN-3-YL)PYRIMIDIN-2-YLAMINO)PHENYL]-BENZAMIDE
- IMATINIB
Example 10
4.l(4-Methyl-1
-piperazinyl)methyl]-N-[4-methyl-3-[ [4-(3-pyridinyl)-2-
pyrimidinyl]amino]phenyl]- benzamide, pamoate
A
mixture of 4-[(4-methyl-1- piperazinyl)
methyl]-N-[4-methyl-3-[[4-(3-pyridinyl)-2- pyrimidinyl]amino] phenyl]-benzamide
(4.94 g, 10 mmol) and 4,4'-methylenebis[3-hydroxy-2- naphthoic acid (Fluke,
Buchs, Switzerland; 3.88 g, 10 mmol) in ethanol (50 mL) is heated.
Water (25 mL) is then added. Upon cooling, the product crystallizes and is
filtered-off and dried to afford 4-[(4-methyl-1- piperazinyl)methyl]-N-
[4-methyl-3-[[4-(3-pyridinyl)- 2- pyrimidinyl]amino]phenyl]-benzemide, pamoate
as a pale- yellow solid, having the following analytical properties: Analysis
found: C, 69.12; H. 5.62; N. 10.88%; H2O, 2.50%. Calculated for C52H47N7O7-
1.26 H2O: C, 69.04; H. 5.52; N. 10.84%; H2O, 2. 51%.
Heating
pamoic acid in ethanol will create some solubility. The solids must have
dissolved since the addition of water is usually done to the point of turbidity
and then the crystals allowed to come out as the solution cools.
WO05012233A1
MELDONIUM
SALTS, METHOD OF THEIR PREPARATION AND PHARMACEUTICAL COMPOSITION ON THEIR
BASIS (CH3)3N+-NHCH2CH2COOH
X-
EXAMPLE
10
Meldonium pamoate (1:1; x H20). Meldoniurn (5.46 g, 30 mmol) and pamoic acid
(5.82 g, 15 mmol) are mixed with water and acetone (15 ml), the formed
suspension is evaporated, 30-40 ml toluene is added to the residual viscous
mass, it is grated, and evaporation is repeated. If the residue is
insufficiently dry, treatment with toluene is repeated. Mp. 128-133°C
(decomp.). H NMR spectrum (DMSO-d6), 6, ppm: 2.41 (2H, t, CH2COO-); 3.14 (2H,
t, CH2N); 3.25 (9H, s, Me3N+); 4.75 (2H, s, -CH=(pam)) , 7.12 (2H, t, Harom);
7.26 (2H, td, Harom); 7.77 (2H, d, Harom); 8.18 (2H, d, Harom); 8.35 (2H, s,
Harom). Found, %: C 62,90; H 5,83; N 4,98. Calculated, %: C 63,07; H S,84; N
5,07. Initially H:O content in the sample was 1.71%; after 24 hours maintenance
at 100% humidity sample mass increased by 9% due to absorbed water.
Pamoic
acid is not particularly soluble in either water or acetone. Evaporation would readily remove the acetone.
The water would only be grudgingly removed as an azeotrope with toluene.
WO0008016A1
PAROXETINE
SALTS
Example
32 : Preparation of paroxetine pamoate 1: 1 salt.
A solution of paroxetine base in toluene
(5 ml, 2. 10 g) was added to a solution of pamoic acid (2.48 g) in pyridine (40
ml), and the mixture was stirred at ambient temperature for 30 minutes. The
solvent was then removed by distillation at reduced pressure, the residual oil
diluted with toluene (30 ml) and the solvent again removed by distillation at
reduced pressure. This procedure was repeated two more times. The solid product
was washed with hot diethylether (c. 100 ml x 3) , and filtered under nitrogen
to give a pale yellow solid. The product was washed twice more with diethylether (2 x 100 n- A), and then with methanol (30 ml), and finally dried under
vacuum.
Yield = 3.27 g,
IR nujol mull:
Bands at 1636, 1558, 1508, 1459, 1377,
1183, 1036, 830, 722 CM-1.
Example 33 : Preparation of paroxetine pamoate
2:1 salt
.
A solution of paroxetine base in toluene
(10 ml, 4.2 g) was added to a solution of pamoic acid (2.48 g) in pyridine (40
ml). The mixture was stirred at ambient temperature for 30 minutes. The solvent
was then removed by distillation at reduced pressure, the residual oil diluted
with toluene (30 ml) and the solvent again removed by distillation at reduced
pressure. This procedure was repeated two more times. The solid product was
washed with diethyl ether (c. 50 ml), and filtered under nitrogen to give a
white solid. This solid was washed twice more with diethyl ether (2 x 10 ml),
and then dried under vacuum.
Yield 6.7 g.
IR nujol mull:
Bands at 1641, 1461, 13 77, 1181, 1035,
829, 757 cm- 1.
Pamoic
acid is soluble in pyridine presumably as a pyridinium salt. It can be
recrystallized from dilute aqueous pyridine.
It is also soluble in nitrobenzene.
Embonic
acid has been used to precipitate amines from a heterogeneous natural product
extract or from reaction mixtures, which may contain considerable quantities of
unwanted organic matter as well as inorganic salts. When htis is the case,
Barber & Gaimster recommend that the crystallization of the embonates can
often be facilitated by the addition of acetone, to the extent of 10 to 15% of
the total volume.
Molecules
2007, 121313
Extraction
and precipitation of alkaloid-embonates
Homogenous
dried leaves of a registered Finnish variety of C. roseus (1.0 g) were
extracted for 30minutes with 0.1 M hydrochloric acid solution (100 mL) in an
ultrasonic bath (USF Finnsonic W 181, Ultra Sonic Finland). The mixture was
then centrifuged at 2000 rpm for 10 min and the sediment wasre-extracted with
additional HCl (100 mL) for another 30 minutes. The combined supernatant from
two repeated extractions was filtered and extracted with petroleum ether (200
mL) to eliminate chlorophyll and other lipophilic compounds. The acidic
fraction was separated and an alkaline solution (pH 10.5) of 10 % embonic acid
was slowly added for the precipitation of alkaloids as their embonate
complexes. The pH of the resultant solution was increased to 5.0. The
precipitate was separated simply by decantation and it was used as starting
material for the semi-synthesis.
Based
on the forgoing information, Kilomentor would like to suggest that a basic
process intermediate could be highly likely to be precipitated either (i) from
a reaction mixture simply by the addition of a hot ethanolic solution of
embonic acid or (ii) from the aqueous acid extract of the reaction mixture by
the addition of a solution of sodium embonate.
Higher molecular weight amines are generally more likely to precipitate
than those of lower molecular weight and dibasic molecules are more likely to
precipitate than monobasic ones.
The
methodology could also possibly be used to recover reagents tagged with basic
amino groups by extracting them from neutral reaction mixtures and then
precipitating the embonates of the reagents, thus recovering them for
recycling. If it were to turn out that the corresponding embonate salts were
insoluble, one can imagine using this method to recover such useful but
expensive basic reagents as diazabicyclooctane (DABCO), diazabicyclononane
(DBN), diazabicycloundecane (DBU) tetramethylethylenediamine, ethyl
dicyclohexylamine, ethyl diisopropylamine, tributylamine,
tris(2-hydroxypropyl)amine, 2,2,6,6-tetramethylpiperidine,
1,2,2,6,6-pentamethylpiperidine, tetramethylguanidine,
1,8-bis-dimethylamno-naphthalene, dicyclohexylamine, ethyl
3,3-dimethylaminopropylcarbodiimide.
Cationic
phase transfer catalysts could possibly be recovered as insoluble
embonates.
The
reagent N-benzyl-N,N-dimethylaniline hydroxide is used to benzylate free
carboxylics by refluxing in a high boiling solvent to give the benzyl ester and
dimethylaniline. The reagent is presently prepare by treating
N-benzyl-N,N-dimethylaniline halide with silver oxide. It would seem to be less
expensive and more convenient to precipitate the embonate and cleave it with
sodium hydroxide.
The
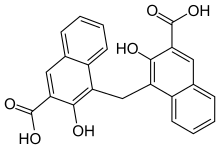
article by Barber and Gaimster, J. appl. Chem.,2, October, 1952. teaches another
easily synthesized diacid structure that can give highly insoluble salts. This
new acid , 2:2’-dihydroxy-1:1’-dinaphthyl-3:3’-dicarboxylic acid. It differs
from embonic acid in that the single methylene, which connects the two naphthyl
groups in embonic acid has been replaced by a direct connection between the
rings.
The
compound is made according to the following procedure”
2-Hydroxy-3-naphthoic
acid (18.8g.) was dissolved in a solution of sodium hydroxide (8 g.) in water
(580 ml.) and the solution was refluxed while a solution of ferric chloride (23
g. of the hexahydrate) and concentrated hydrochloric acid (26 ml) in water (29
ml.) was added drop-wise with strong stirring during 30 minutes, then cooled,
filtered and the filtrate rejected. After washing with a little water, the
residue was dissolved in a slight excess of N-sodium hydroxide solution (about
200 ml.). The solution was treed with charcoal, filtered, acidified with
concentrated hydrochloric acid and filtered.
The yellow residue, after washing with water, was recrystallized from
aqueous ethanol to give 2.8 g. , 2:2’-dihydroxy-1:1’-dinaphthyl-3:3’-dicarboxylic
acid as a pale-yellow hemihydrate, mp 330-333 C.